84 l © 2025 American Dental Association
Section 3: Fluoridation Practice
49. Does fluoridating the community water supply raise concerns about
lead, arsenic, and other toxic contaminants to the water supply?
Answer
No. The concentrations of contaminants in drinking water as a result of fluoridation do not exceed
and are in fact well below regulatory standards set to ensure the public’s safety.
Fact
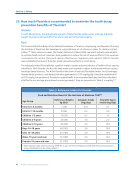
Fluorosilicic acid is used to fluoridate the majority of community water systems in the United States.89
Because the additive is derived from ore mined from the Earth, fluorosilicic acid can contain minute
amounts of contaminants, such as lead and arsenic. However, existing regulations and standards
require that these contaminants, and others, be at levels considered acceptable by the EPA when
fluorosilicic acid or other fluoridation additives are diluted to produce optimally fluoridated water.365
NSF/ANSI Standard 60, as well as AWWA standards, are applicable to all fluoride additives.36,365
Testing of fluoride additives provides evidence that the levels of these contaminants do not exceed
and are in fact well below regulatory standards set to ensure the public’s safety. NSF has prepared
a detailed fact sheet, NSF Fact Sheet on Fluoridation Products,90 that provides the documented
quality of fluoride additives based on product samples analyzed. The NSF reports that the majority
of fluoridation products as a class, based on NSF test results, do not add measurable amounts of
arsenic, lead, or other heavy metals or radionuclides to drinking water.90,91
50. Have fluoride additives been tested for safety?
Answer
The claim is sometimes made that no studies on safety exist on the additives used in water fluoridation.
This statement is misleading because the scientific community does not study the health effects of
the concentrated additives studies are done on the health effects of the treated water.
Fact
A 1999 study345 charged that fluorosilicic acid and sodium silicofluoride did not disassociate (break
down) completely when added to water systems and could be responsible for lower pH (acid) levels of
drinking water, leaching lead from plumbing systems and increasing lead uptake by children. Scientists
from the EPA evaluated the disassociation of fluoride additives346 and concluded that at the typical pH
level of drinking water (which is normally slightly alkaline) and the fluoride levels used in drinking water,
the fluoride additives quickly and completely broke down to fluoride ions and silica.
University of Michigan Study
In 2006,347 researchers at the University of Michigan verified for the EPA that theoretical predictions
that hexafluorosilicate completely hydrolyzed (broke down) when added to water, separating into
free fluoride ions and silica ions, were confirmed. The research demonstrated that there was no
hexafluorosilicate that could be measured in the finished water.347
Silicofluoride
While sodium fluoride was the first additive used in water fluoridation, the use of silicofluoride additives
(sodium fluorosilicate and fluorosilicic acid) began in the late 1940s. By 1951, silicofluorides had
become the most commonly used fluoride additives in water fluoridation.88 Many of the early studies
Section 3: Fluoridation Practice
49. Does fluoridating the community water supply raise concerns about
lead, arsenic, and other toxic contaminants to the water supply?
Answer
No. The concentrations of contaminants in drinking water as a result of fluoridation do not exceed
and are in fact well below regulatory standards set to ensure the public’s safety.
Fact
Fluorosilicic acid is used to fluoridate the majority of community water systems in the United States.89
Because the additive is derived from ore mined from the Earth, fluorosilicic acid can contain minute
amounts of contaminants, such as lead and arsenic. However, existing regulations and standards
require that these contaminants, and others, be at levels considered acceptable by the EPA when
fluorosilicic acid or other fluoridation additives are diluted to produce optimally fluoridated water.365
NSF/ANSI Standard 60, as well as AWWA standards, are applicable to all fluoride additives.36,365
Testing of fluoride additives provides evidence that the levels of these contaminants do not exceed
and are in fact well below regulatory standards set to ensure the public’s safety. NSF has prepared
a detailed fact sheet, NSF Fact Sheet on Fluoridation Products,90 that provides the documented
quality of fluoride additives based on product samples analyzed. The NSF reports that the majority
of fluoridation products as a class, based on NSF test results, do not add measurable amounts of
arsenic, lead, or other heavy metals or radionuclides to drinking water.90,91
50. Have fluoride additives been tested for safety?
Answer
The claim is sometimes made that no studies on safety exist on the additives used in water fluoridation.
This statement is misleading because the scientific community does not study the health effects of
the concentrated additives studies are done on the health effects of the treated water.
Fact
A 1999 study345 charged that fluorosilicic acid and sodium silicofluoride did not disassociate (break
down) completely when added to water systems and could be responsible for lower pH (acid) levels of
drinking water, leaching lead from plumbing systems and increasing lead uptake by children. Scientists
from the EPA evaluated the disassociation of fluoride additives346 and concluded that at the typical pH
level of drinking water (which is normally slightly alkaline) and the fluoride levels used in drinking water,
the fluoride additives quickly and completely broke down to fluoride ions and silica.
University of Michigan Study
In 2006,347 researchers at the University of Michigan verified for the EPA that theoretical predictions
that hexafluorosilicate completely hydrolyzed (broke down) when added to water, separating into
free fluoride ions and silica ions, were confirmed. The research demonstrated that there was no
hexafluorosilicate that could be measured in the finished water.347
Silicofluoride
While sodium fluoride was the first additive used in water fluoridation, the use of silicofluoride additives
(sodium fluorosilicate and fluorosilicic acid) began in the late 1940s. By 1951, silicofluorides had
become the most commonly used fluoride additives in water fluoridation.88 Many of the early studies