© 2025 American Dental Association l 45
Fluoridation Facts
was 36.7 years), even at these levels more than 10 times higher than recommended for tooth decay
prevention, resulted in no clinically significant physiological or functional effects.186
Fluoride in Food and Beverages
In looking at the fluoride content of food and beverages over time, it appears that fluoride intake from
dietary sources has remained relatively constant.207 Except for products prepared (commercially or by
the individual) or cooked with fluoridated water, the fluoride content of most food and beverages is not
significantly different between fluoridated and non-fluoridated communities. When fluoridated water
is used to prepare or cook the samples, the fluoride content of food and beverages is higher.205,207
While information on the fluoride content of food and beverages, including the USDA’s National
Fluoride Database,206 is limited and may not reflect current levels, in general, the fluoride content
of fresh solid food in the United States is relatively low and ranges from 0.01 to 1.0 ppm.207 The
foods highest in fluoride are fish and shellfish, reflective of the fluoride found in ocean water and
the presence or absence of bone fragments, such as those in sardines.207 (Fluoride has an affinity for
calcified tissues such as bones.) Cereals, baked goods, breads, and other grain products generally
have fluoride concentrations between 0.06 and 0.72 ppm.207 The majority of vegetables (leafy, root,
legumes, green, or yellow) have a relatively low fluoride concentration (ranging from 0.01 to 0.5 ppm),
with fruits generally having lower concentrations (ranging from 0.01 to 0.2 ppm) than vegetables.207
Raisins are one exception in the fruit category, with a higher fluoride concentration due to the use
of certain pesticides and concentrations of fluoride through drying.207 Brewed teas generally contain
fluoride concentrations of 1–6 ppm, depending on the amount of dry tea used, the water fluoride
concentration, and the brewing time.208 Note that the fluoride concentration of unsweetened instant
tea powder appears very high when reported as a dry powder because this product is extremely
concentrated. However, when one teaspoon of the unsweetened tea powder is added to an 8-ounce cup
of water, the concentration for prepared instant tea is similar to that reported for regular brewed tea.206
Alcoholic beverages also contain fluoride and the amount varies, with distilled spirits showing the lowest
average levels at 0.08 ppm, beer at 0.45 ppm, red wine at about 1 ppm, and white wine at about 2 ppm.206
Food and beverages that are processed commercially in cities fluoridated to the recommended level
generally contain higher levels of fluoride than those processed in non-fluoridated communities.
These foods and beverages are consumed not only in the city where they are processed, but also
often are distributed to and consumed in non-fluoridated areas.58 This “halo” or “diffusion” effect
results in increased fluoride intake by people in non-fluoridated communities, providing them
increased protection against tooth decay.105,209
Also, most people in both water-fluoridated and non-fluoridated communities use fluoride toothpaste.
As a result of the widespread availability of these various sources of fluoride, the differences between
tooth decay rates in fluoridated areas and non-fluoridated areas are somewhat less than they were
many decades ago, but these differences are still substantial. Failure to account for the diffusion
effect results in an underestimation of the total benefit of water fluoridation.105
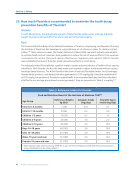
The average daily dietary intake of fluoride (expressed on a body weight basis) by children residing in
communities with water fluoridated at 1.0 mg/L has been found to be about 0.05 milligrams per kilogram
of body weight per day (mg/kg/day).146 In communities without optimally fluoridated water, average intakes
for children were about 50% lower.146 Dietary fluoride intake by adults in communities where water was
fluoridated at 1.0 mg/L averaged 1.4–3.4 mg/day, and in non-fluoridated areas it averaged 0.3–1.0 mg/
day.146 With the current recommendation that drinking water be fluoridated at 0.7 mg/L, average intakes
would be about 30% lower in fluoridated communities than when they were fluoridated at 1.0 mg/L.
Fluoridation Facts
was 36.7 years), even at these levels more than 10 times higher than recommended for tooth decay
prevention, resulted in no clinically significant physiological or functional effects.186
Fluoride in Food and Beverages
In looking at the fluoride content of food and beverages over time, it appears that fluoride intake from
dietary sources has remained relatively constant.207 Except for products prepared (commercially or by
the individual) or cooked with fluoridated water, the fluoride content of most food and beverages is not
significantly different between fluoridated and non-fluoridated communities. When fluoridated water
is used to prepare or cook the samples, the fluoride content of food and beverages is higher.205,207
While information on the fluoride content of food and beverages, including the USDA’s National
Fluoride Database,206 is limited and may not reflect current levels, in general, the fluoride content
of fresh solid food in the United States is relatively low and ranges from 0.01 to 1.0 ppm.207 The
foods highest in fluoride are fish and shellfish, reflective of the fluoride found in ocean water and
the presence or absence of bone fragments, such as those in sardines.207 (Fluoride has an affinity for
calcified tissues such as bones.) Cereals, baked goods, breads, and other grain products generally
have fluoride concentrations between 0.06 and 0.72 ppm.207 The majority of vegetables (leafy, root,
legumes, green, or yellow) have a relatively low fluoride concentration (ranging from 0.01 to 0.5 ppm),
with fruits generally having lower concentrations (ranging from 0.01 to 0.2 ppm) than vegetables.207
Raisins are one exception in the fruit category, with a higher fluoride concentration due to the use
of certain pesticides and concentrations of fluoride through drying.207 Brewed teas generally contain
fluoride concentrations of 1–6 ppm, depending on the amount of dry tea used, the water fluoride
concentration, and the brewing time.208 Note that the fluoride concentration of unsweetened instant
tea powder appears very high when reported as a dry powder because this product is extremely
concentrated. However, when one teaspoon of the unsweetened tea powder is added to an 8-ounce cup
of water, the concentration for prepared instant tea is similar to that reported for regular brewed tea.206
Alcoholic beverages also contain fluoride and the amount varies, with distilled spirits showing the lowest
average levels at 0.08 ppm, beer at 0.45 ppm, red wine at about 1 ppm, and white wine at about 2 ppm.206
Food and beverages that are processed commercially in cities fluoridated to the recommended level
generally contain higher levels of fluoride than those processed in non-fluoridated communities.
These foods and beverages are consumed not only in the city where they are processed, but also
often are distributed to and consumed in non-fluoridated areas.58 This “halo” or “diffusion” effect
results in increased fluoride intake by people in non-fluoridated communities, providing them
increased protection against tooth decay.105,209
Also, most people in both water-fluoridated and non-fluoridated communities use fluoride toothpaste.
As a result of the widespread availability of these various sources of fluoride, the differences between
tooth decay rates in fluoridated areas and non-fluoridated areas are somewhat less than they were
many decades ago, but these differences are still substantial. Failure to account for the diffusion
effect results in an underestimation of the total benefit of water fluoridation.105
The average daily dietary intake of fluoride (expressed on a body weight basis) by children residing in
communities with water fluoridated at 1.0 mg/L has been found to be about 0.05 milligrams per kilogram
of body weight per day (mg/kg/day).146 In communities without optimally fluoridated water, average intakes
for children were about 50% lower.146 Dietary fluoride intake by adults in communities where water was
fluoridated at 1.0 mg/L averaged 1.4–3.4 mg/day, and in non-fluoridated areas it averaged 0.3–1.0 mg/
day.146 With the current recommendation that drinking water be fluoridated at 0.7 mg/L, average intakes
would be about 30% lower in fluoridated communities than when they were fluoridated at 1.0 mg/L.