58 l © 2025 American Dental Association
Section 2: Safety
Drinking Water
As stated in Question 27, in 2015 the USPHS established a nation-wide recommended level of fluoride
for water fluoridation (at 0.7 mg/L) to provide the best balance of protection from tooth decay while
reducing the risk for dental fluorosis.39 While it is too early to determine the impact of this change on
the prevalence and severity of dental fluorosis, studies in other countries where optimal water fluoride
levels were reduced showed lower fluorosis prevalence.269
In areas where naturally occurring fluoride levels in ground water are high (e.g., 2 mg/L or higher),
the EPA recommends consumers consider alternative sources for drinking water for young children.204
Families with young children on community water systems should check their Consumer Confidence
Reports on water quality or contact their water suppliers to ask about the fluoride level in their drinking
water. Consumers with private wells should have the water tested yearly to accurately determine the
fluoride content. Consumers should consult with their dentist regarding water-testing results and discuss
appropriate dental health preventive measures.204
Infant Formula
As detailed in Question 28, promoting breastfeeding is consistent with guidelines for infant nutrition,
and breastfeeding of more than 6 months has been shown to reduce the prevalence of dental fluorosis
compared with not breastfeeding.270 Another study demonstrated that fluoride ingestion is significantly
lower when reconstituting infant formula concentrate with bottled water than it is when using tap water.271
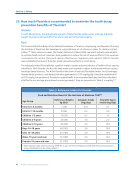
30. Why is there a warning label on a tube of fluoride toothpaste?
Answer
The FDA has established regulations for warning labels for a number of OTC items it considers
safe and effective, including fluoride toothpaste.
Fact
The FDA has published regulations regarding warning labels for OTC drugs in the Code of Federal
Regulations (CFR).272 All non-prescription drugs covered by these regulations must display the general
warning “Keep out of the reach of children” in bold type. The regulations outline three additional warning
statements (based on the most likely route of exposure) to be listed on the label in the event the drug is
misused. While they vary slightly, they all include the following language: “…get medical help or contact
a Poison Control Center right away.”272
In the CFR, the FDA has outlined the drug categories to be covered by these warning labels.273
Some of the 26 categories include antacids, allergy treatment products, antiperspirants, cold
remedies, ophthalmic products, dentifrices (toothpastes), and dental products such as analgesics and
antiseptics.273 A specific FDA regulation274 applies to “Anticaries Drug Products for Over-The-Counter
Human Use,” which provides the exact language for the warning label to be used on “fluoride dentifrice
(gel, paste, and powder) products.” The regulation requires that the following language appear on
these products under the heading “Warning”:
“Keep out of reach of children under 6 years of age. If more than used for brushing is accidentally
swallowed, get medical help or contact a Poison Control Center right away.” 274
The OTC drugs listed in these regulations are generally recognized as safe and effective by the FDA.272
Fluoride toothpaste is just one in a long list of OTC products that carries a warning label.
Section 2: Safety
Drinking Water
As stated in Question 27, in 2015 the USPHS established a nation-wide recommended level of fluoride
for water fluoridation (at 0.7 mg/L) to provide the best balance of protection from tooth decay while
reducing the risk for dental fluorosis.39 While it is too early to determine the impact of this change on
the prevalence and severity of dental fluorosis, studies in other countries where optimal water fluoride
levels were reduced showed lower fluorosis prevalence.269
In areas where naturally occurring fluoride levels in ground water are high (e.g., 2 mg/L or higher),
the EPA recommends consumers consider alternative sources for drinking water for young children.204
Families with young children on community water systems should check their Consumer Confidence
Reports on water quality or contact their water suppliers to ask about the fluoride level in their drinking
water. Consumers with private wells should have the water tested yearly to accurately determine the
fluoride content. Consumers should consult with their dentist regarding water-testing results and discuss
appropriate dental health preventive measures.204
Infant Formula
As detailed in Question 28, promoting breastfeeding is consistent with guidelines for infant nutrition,
and breastfeeding of more than 6 months has been shown to reduce the prevalence of dental fluorosis
compared with not breastfeeding.270 Another study demonstrated that fluoride ingestion is significantly
lower when reconstituting infant formula concentrate with bottled water than it is when using tap water.271
30. Why is there a warning label on a tube of fluoride toothpaste?
Answer
The FDA has established regulations for warning labels for a number of OTC items it considers
safe and effective, including fluoride toothpaste.
Fact
The FDA has published regulations regarding warning labels for OTC drugs in the Code of Federal
Regulations (CFR).272 All non-prescription drugs covered by these regulations must display the general
warning “Keep out of the reach of children” in bold type. The regulations outline three additional warning
statements (based on the most likely route of exposure) to be listed on the label in the event the drug is
misused. While they vary slightly, they all include the following language: “…get medical help or contact
a Poison Control Center right away.”272
In the CFR, the FDA has outlined the drug categories to be covered by these warning labels.273
Some of the 26 categories include antacids, allergy treatment products, antiperspirants, cold
remedies, ophthalmic products, dentifrices (toothpastes), and dental products such as analgesics and
antiseptics.273 A specific FDA regulation274 applies to “Anticaries Drug Products for Over-The-Counter
Human Use,” which provides the exact language for the warning label to be used on “fluoride dentifrice
(gel, paste, and powder) products.” The regulation requires that the following language appear on
these products under the heading “Warning”:
“Keep out of reach of children under 6 years of age. If more than used for brushing is accidentally
swallowed, get medical help or contact a Poison Control Center right away.” 274
The OTC drugs listed in these regulations are generally recognized as safe and effective by the FDA.272
Fluoride toothpaste is just one in a long list of OTC products that carries a warning label.