© 2025 American Dental Association l 5
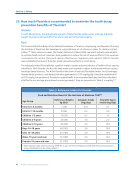
History of Water Fluoridation
2018 Chaitanya NC, Karunakar P, Allam NSJ, Priya MH, Alekhya B, Nauseen S. A systematic analysis
on possibility of water fluoridation causing hypothyroidism. Indian J Dent Res. 2018,29(3),
358-363.33
2017 Australian Government. National Health and Medical Research Council (NHMRC). Information
Paper — Water Fluoridation: Dental and Other Human Health Outcomes.34
2016 O’Mullane DM, Baez RJ, Jones S, Lennon MA, Petersen PE, Rugg-Gunn AJ, Whelton H, Whitford
GM. Fluoride and Oral Health.35
2016 American Water Works Association. Water Fluoridation Principles and Practices. AWWA Manual
M4. Sixth edition.36
2015 Water Research Foundation. State of the Science: Community Water Fluoridation.37
2015 Ireland Health Research Board. Health Effects of Water Fluoridation: An Evidence Review.38
2015 US Department of Health and Human Services Federal Panel on Community Water Fluoridation.
US Public Health Service Recommendation for Fluoride Concentration in Drinking Water for the
Prevention of Dental Caries.39
2014 Public Health England. Water Fluoridation: Health Monitoring Report for England.40
2014 Royal Society of New Zealand and the Office of the Prime Minister’s Chief Science Advisor.
Health Effects of Water Fluoridation: A Review of the Scientific Evidence.41
2013 US Community Preventive Services Task Force. The Guide to Community Preventive Services.
Preventing Dental Caries: Community Water Fluoridation.42
2011 European Commission of the European Union Scientific Committee on Health and Environmental
Risks (SCHER). Fluoridation.43
2008 Health Canada. Findings and Recommendations of the Fluoride Expert Panel.44
2007 Australian Government. National Health and Medical Research Council A Systematic Review
of the Efficacy and Safety of Fluoridation Part A: Review Methodology and Results.45
History of Water Fluoridation
2018 Chaitanya NC, Karunakar P, Allam NSJ, Priya MH, Alekhya B, Nauseen S. A systematic analysis
on possibility of water fluoridation causing hypothyroidism. Indian J Dent Res. 2018,29(3),
358-363.33
2017 Australian Government. National Health and Medical Research Council (NHMRC). Information
Paper — Water Fluoridation: Dental and Other Human Health Outcomes.34
2016 O’Mullane DM, Baez RJ, Jones S, Lennon MA, Petersen PE, Rugg-Gunn AJ, Whelton H, Whitford
GM. Fluoride and Oral Health.35
2016 American Water Works Association. Water Fluoridation Principles and Practices. AWWA Manual
M4. Sixth edition.36
2015 Water Research Foundation. State of the Science: Community Water Fluoridation.37
2015 Ireland Health Research Board. Health Effects of Water Fluoridation: An Evidence Review.38
2015 US Department of Health and Human Services Federal Panel on Community Water Fluoridation.
US Public Health Service Recommendation for Fluoride Concentration in Drinking Water for the
Prevention of Dental Caries.39
2014 Public Health England. Water Fluoridation: Health Monitoring Report for England.40
2014 Royal Society of New Zealand and the Office of the Prime Minister’s Chief Science Advisor.
Health Effects of Water Fluoridation: A Review of the Scientific Evidence.41
2013 US Community Preventive Services Task Force. The Guide to Community Preventive Services.
Preventing Dental Caries: Community Water Fluoridation.42
2011 European Commission of the European Union Scientific Committee on Health and Environmental
Risks (SCHER). Fluoridation.43
2008 Health Canada. Findings and Recommendations of the Fluoride Expert Panel.44
2007 Australian Government. National Health and Medical Research Council A Systematic Review
of the Efficacy and Safety of Fluoridation Part A: Review Methodology and Results.45