© 2025 American Dental Association l 69
Fluoridation Facts
40. For women, does drinking water fluoridated at the recommended
level create a risk for their children to be born with Down syndrome?
Answer
No. There is no known association between the consumption of drinking water fluoridated at
the recommended levels and Down syndrome.
Fact
All people with Down syndrome have an extra, critical portion of chromosome 21 present in all or
some of their cells. This additional genetic material alters the course of development and causes the
characteristics associated with Down syndrome. The cause of the extra full or partial chromosome
is still unknown. Maternal age is the major factor that has been linked to an increased chance of
having a baby with Down syndrome. There is no definitive scientific research that indicates that Down
syndrome is caused by environmental factors or the parents’ activities before or during pregnancy.317
A number of studies have looked at this issue and several are summarized here.
US Studies
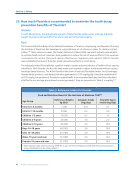
A detailed study318 of approximately 2,500 children born with Down syndrome was conducted in
Massachusetts. A rate of 1.5 cases per 1,000 births was found in both fluoridated and non-fluoridated
communities, providing strong evidence that fluoridation does not increase the risk of Down syndrome.318
A comprehensive study of Down syndrome births was conducted in 44 US cities over a two-year
period. Rates of Down syndrome were comparable in fluoridated and non-fluoridated cities.319
United Kingdom (UK) Studies
In 2014, a systematic review published by Public Health England reviewed the literature and concluded
there was no evidence of a difference in the rate of Down syndrome in fluoridated and non-fluoridated
areas.40
Another large population-based study examined trends in births before and after fluoridation in
the Birmingham. The study showed no association between water fluoridation and the incidence of
congenital malformations, including Down syndrome.320
Rates of Down syndrome were comparable in fluoridated and non-fluoridated cities.319
Fluoridation Facts
40. For women, does drinking water fluoridated at the recommended
level create a risk for their children to be born with Down syndrome?
Answer
No. There is no known association between the consumption of drinking water fluoridated at
the recommended levels and Down syndrome.
Fact
All people with Down syndrome have an extra, critical portion of chromosome 21 present in all or
some of their cells. This additional genetic material alters the course of development and causes the
characteristics associated with Down syndrome. The cause of the extra full or partial chromosome
is still unknown. Maternal age is the major factor that has been linked to an increased chance of
having a baby with Down syndrome. There is no definitive scientific research that indicates that Down
syndrome is caused by environmental factors or the parents’ activities before or during pregnancy.317
A number of studies have looked at this issue and several are summarized here.
US Studies
A detailed study318 of approximately 2,500 children born with Down syndrome was conducted in
Massachusetts. A rate of 1.5 cases per 1,000 births was found in both fluoridated and non-fluoridated
communities, providing strong evidence that fluoridation does not increase the risk of Down syndrome.318
A comprehensive study of Down syndrome births was conducted in 44 US cities over a two-year
period. Rates of Down syndrome were comparable in fluoridated and non-fluoridated cities.319
United Kingdom (UK) Studies
In 2014, a systematic review published by Public Health England reviewed the literature and concluded
there was no evidence of a difference in the rate of Down syndrome in fluoridated and non-fluoridated
areas.40
Another large population-based study examined trends in births before and after fluoridation in
the Birmingham. The study showed no association between water fluoridation and the incidence of
congenital malformations, including Down syndrome.320
Rates of Down syndrome were comparable in fluoridated and non-fluoridated cities.319