© 2025 American Dental Association l 29
Fluoridation Facts
13. The ADA Dietary Fluoride Supplement Schedule from 2010 contains
the word “none” in specific boxes. Does this mean the ADA does not
recommend fluoride for children?
Answer
No, that would be a misinterpretation of the purpose of the schedule. The schedule reflects
the recommended dosage of dietary fluoride supplements based on age and the fluoride level
of the child’s primary source of drinking water, in addition to what would be consumed from
other sources.
Fact
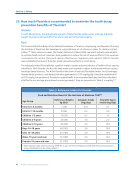
The dietary fluoride supplement schedule75 (Table 1) is just that–a supplement dosage schedule.
Children residing in areas where the drinking water is not fluoridated will receive some fluoride from
other sources, such as foods and beverages. Dietary fluoride supplements are designed for children
over 6 months of age who do not receive a sufficient amount of fluoride from those sources. The
dosage amounts in Table 1 reflect the additional amount of supplemental fluoride intake necessary
to achieve an optimal anti-cavity effect. To reduce the risk of dental fluorosis, children under 6 months
of age should not take dietary fluoride supplements.
Additional information on this topic can be found in the Safety Section, Question 29.
The dietary fluoride supplement schedule should not be viewed as a recommendation of the absolute
upper limits of the amount of fluoride that should be ingested each day. In 2011, the Food and
Nutrition Board of the Institute of Medicine (now National Academies of Sciences, Engineering, and
Medicine) developed Dietary Reference Intakes, a comprehensive set of reference values for dietary
nutrient values. The values present nutrient requirements to optimize health and, for the first time,
set maximum-level guidelines to reduce the risk of adverse effects from excessive consumption
of nutrients. In the case of fluoride, levels were established to reduce tooth decay without causing
moderate dental fluorosis.146
For example, the dietary fluoride supplement schedule recommends that a 2-year-old child at high
risk for tooth decay living in a non-fluoridated area (where the primary water source contains less
than 0.3 ppm fluoride) should receive 0.25 mg of supplemental fluoride per day. This does not mean
that this child should ingest exactly 0.25 mg of fluoride per day total. Instead, a two-year-old child
could receive important anti-cavity benefits by taking 0.25 mg of supplemental fluoride a day without
causing any adverse effects on health. This child would most probably be receiving fluoride from other
sources (other beverages and foods), even in a non-fluoridated area, and the recommendation of
0.25 mg of fluoride per day takes this into account. In the unlikely event that the child did not receive
any additional fluoride from food and beverages, the 0.25 mg per day could be inadequate fluoride
supplementation to achieve an optimal anti-cavity effect.
Additional information on this topic can be found in the Safety Section, Question 23.
It is important to note that the dietary fluoride supplement dosage schedules have been lowered in
the past as exposure to fluoride from other sources has increased. Rather than being a problem, as
those opposed to the use of fluoride might imply, this is evidence that ADA policy is based on the best
available science. Specifically, the ADA periodically reviews the dosage schedule and, working with
other national groups, issues updated recommendations based on the best available science.
Fluoridation Facts
13. The ADA Dietary Fluoride Supplement Schedule from 2010 contains
the word “none” in specific boxes. Does this mean the ADA does not
recommend fluoride for children?
Answer
No, that would be a misinterpretation of the purpose of the schedule. The schedule reflects
the recommended dosage of dietary fluoride supplements based on age and the fluoride level
of the child’s primary source of drinking water, in addition to what would be consumed from
other sources.
Fact
The dietary fluoride supplement schedule75 (Table 1) is just that–a supplement dosage schedule.
Children residing in areas where the drinking water is not fluoridated will receive some fluoride from
other sources, such as foods and beverages. Dietary fluoride supplements are designed for children
over 6 months of age who do not receive a sufficient amount of fluoride from those sources. The
dosage amounts in Table 1 reflect the additional amount of supplemental fluoride intake necessary
to achieve an optimal anti-cavity effect. To reduce the risk of dental fluorosis, children under 6 months
of age should not take dietary fluoride supplements.
Additional information on this topic can be found in the Safety Section, Question 29.
The dietary fluoride supplement schedule should not be viewed as a recommendation of the absolute
upper limits of the amount of fluoride that should be ingested each day. In 2011, the Food and
Nutrition Board of the Institute of Medicine (now National Academies of Sciences, Engineering, and
Medicine) developed Dietary Reference Intakes, a comprehensive set of reference values for dietary
nutrient values. The values present nutrient requirements to optimize health and, for the first time,
set maximum-level guidelines to reduce the risk of adverse effects from excessive consumption
of nutrients. In the case of fluoride, levels were established to reduce tooth decay without causing
moderate dental fluorosis.146
For example, the dietary fluoride supplement schedule recommends that a 2-year-old child at high
risk for tooth decay living in a non-fluoridated area (where the primary water source contains less
than 0.3 ppm fluoride) should receive 0.25 mg of supplemental fluoride per day. This does not mean
that this child should ingest exactly 0.25 mg of fluoride per day total. Instead, a two-year-old child
could receive important anti-cavity benefits by taking 0.25 mg of supplemental fluoride a day without
causing any adverse effects on health. This child would most probably be receiving fluoride from other
sources (other beverages and foods), even in a non-fluoridated area, and the recommendation of
0.25 mg of fluoride per day takes this into account. In the unlikely event that the child did not receive
any additional fluoride from food and beverages, the 0.25 mg per day could be inadequate fluoride
supplementation to achieve an optimal anti-cavity effect.
Additional information on this topic can be found in the Safety Section, Question 23.
It is important to note that the dietary fluoride supplement dosage schedules have been lowered in
the past as exposure to fluoride from other sources has increased. Rather than being a problem, as
those opposed to the use of fluoride might imply, this is evidence that ADA policy is based on the best
available science. Specifically, the ADA periodically reviews the dosage schedule and, working with
other national groups, issues updated recommendations based on the best available science.